Webinars
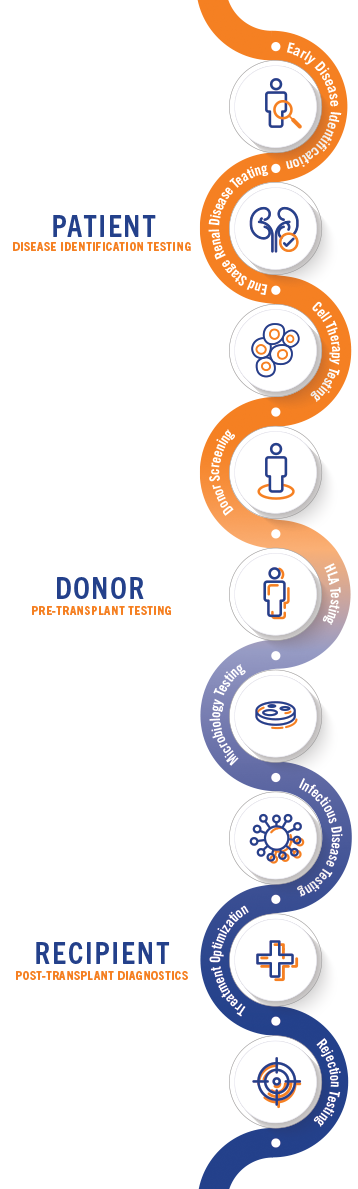
From Pre-Transplant to Post Recovery,
We’re Your Partner Through All of It.

Welcome to the Eurofins Viracor podcast series, your gateway to the latest in transplant science and diagnostics. Tune in as we explore cutting-edge scientific advances, essential diagnostic testing, and the most recent innovations shaping the future of transplant care. Whether you're a clinician, researcher, recipient or healthcare professional, our episodes are designed to keep you informed and inspired. Stay ahead of the curve with insights from leading experts in the field and patient experiences.
Ebook
Learn more about the different companies inside Eurofins Transplant

Case Studies
Explore case studies featuring CMV inSIGHT™ T Cell Immunity testing.

Evaluate Neutropenic Transplant Recipient with CMV inSIGHT™ T Cell Immunity
Molecular Biomarkers Guide Treatment and Prevent Rejection in Graft Monitoring.

Long-Term Monitoring of Graft Health
Whitepapers
Discover how TRAC ID is transforming post-transplant monitoring.